标签:规模 cross 数据分析 arm zed 概率 记忆 新一代 传统
自2015年1月20日美国总统奥巴马高调宣布启动“精准医疗计划(Precision Medicine Initiative)”以来,全球范围内掀起一股精准医疗热。在国内,精准医疗也风生水起,受到业内学者、药企代表、患者等相关人群的广泛关注。精准医疗本质上是一种更为精确的个性化医疗,非常适用于恶性肿瘤的临床治疗。而相对于其他精准医疗策略,精准细胞免疫治疗(precision cell immunotherapy,PCIT)具有开发周期相对较短、投入相对较低的优势,适合我国的国情,具有巨大的应用前景,有望成为我国恶性肿瘤精准医疗的一大突破口。
精准医疗(precision medicine)是通过基因组、蛋白质组等组学技术和医学前沿技术,对疾病进行精细分类及精确诊断,从而对疾病和特定患者进行个性化精准疗的新型医学概念与医疗模式。2011年,在“人类基因组计划”完成近10年后,这一概念由美国著名基因组学家Olson博士在其参与起草的美国国家智库报告《走向精准医疗》中首次提出。精准医疗模式集合了诸多现代医学科技发展的知识与技术体系,体现了医学科学发展趋势,也代表了临床实践发展的方向,将带来一场新的医疗革命并将深刻响未来医疗模式。正是基于此考虑,2015年1月20日,美国总统奥巴马在白宫高调宣布启动“精准医疗计划”,拟通过分析100万名志愿者的基因信息,研究遗传性变异在疾病发生发展中的作用,了解疾病治疗的分子基础,为药物研发与患者“精准治疗”明确方向,以推动个性化医疗的发展,并希望以此“引领一个医学新时代”。
在美国提出的精准医疗计划中,恶性肿瘤的精准医疗是“重中之重”。美国国立卫生研究院下设的国家癌症研究所,将接受重点资助开展解码肿瘤基因及开发精准治疗研究。那么,为什么要从肿瘤着手开展精准医疗计划呢?诚然,这与当前日趋严峻的肿瘤防治形势相关,另一个重要的原因是提升 肿瘤疗效的迫切需求。众所周知,肿瘤本质上是一种由一系列基因变异的积累导致的复杂遗传疾病,这意味着肿瘤的基因组是动态变化的,且存在着高度异质性。不同的疾病进展阶段以及不同的肿瘤细胞可能携带不同的变异信息,从而对以大规模人群为基础开发和测试药物的治疗模式构成了颠覆性挑战。据一项覆盖9个国家和地区的1217例患者的泛亚洲科研显示:如果没有基因检测鉴定相关的靶标却接受了靶向治疗,死亡风险将增加185%。 而新一代测序技术能够无假设、高分辨率地分析基因组,获知这些不同的变异信息,能为制定更具针对性和有效性的防治措施提供准确依据,指导医生对患者采取个性化用药。基于上述原因,精准医疗模式已然成为癌症治疗刻不容缓的任务,是恶性肿瘤治疗的大势所趋。
在具体操作中,肿瘤精准医疗通常可划分为以下三部曲:基因检测,大数据分析和用药指导。第一步,基因检测是患者变异信息的获知过程,如通过高通量测序方法获得肿瘤单核苷酸有义突变、拷贝数变异、基因移位和融合基因等海量基因变异信息,该环节中相关检测技术的精确性及所检测对象(如肿瘤组织标本)所反映信息的全面性是关键。第二步,大数据分析是相关变异信息的解码与提炼过程,即从海量的组学数据中抽丝剥茧、去粗存精,提取有 价值信息,发挥前后两个环节之间承上启下的作用,该环节相应分析模型与分析方法的精确性是关键。第三步,用药指导是以大数据分析结果作为参考,制定因人因病而异的治疗方案的过程;而治疗的结果也可以反馈到第一个环节,通过新的环路保证治疗能随病情的变化而做出相应的调整。候选药物可涵盖所有类型恶性肿瘤临床用药,甚至用于其他疾病治疗的药物。该环节中,可供选择的治疗药物的丰富度直接关系到实施精准医疗治疗的成败。
精准医疗直指恶性肿瘤临床治疗的软肋,其好处不言而喻,已在临床治疗中越来越显现其价值。 然而,每个患者多个癌细胞在癌变过程中与之相关的基因突变位点有成千上万处,而其中起决定性作用的基因突变往往不足十处,如何从每个患者成千上万处体细胞突变中找到每个肿瘤细胞真正的阿喀琉斯之踵,即引发癌变的关键基因,并非是一件容易的事;由于肿瘤的异质性,同一肿瘤患者不同癌细胞的基因突变并不一定相同,不同关键基因突变的随机组合,导致癌症治疗难度大为增加;更为严重的事是癌症细胞周期检查点已破坏,各种新突变及融合基因仍在不断累积,这些新突变及融合基因可能会破坏这些靶向药物的靶点及其下游信号,从而使靶向治疗药物失效。因此,看似已抑制了关键基因,但癌症细胞又建立新的关键基因并产生旁路。按精准医学模式,希望将癌症变为一种慢性病,但从发现靶点—使用靶向药物—靶点突变或建立新旁路—癌症复发—寻找新靶点—使用新靶向药物,这种反复的猫捉老鼠的游戏,传统药物开发手段难以开发满足所有变异信息的治疗药物,同时肿瘤基因突变的速度可导致费尽心思寻找到的药物在几个月时间内失效,患者只能辗转于不同药物的变换,对患者家庭乃至整个医疗保险体系造成巨大经济负担。肿瘤精准医疗的这一系统性缺陷应值得引起充分的重视。
人类的免疫系统具有高度的特异性,能正确区分 正常和恶性细胞,能以高度的敏感性和特异性识别 “非自我的”分子或细胞,功能正常的T淋巴细胞能 通过其细胞表面TCR受体(T cell receptor)正确识别 肿瘤细胞中“非自我”改变,清除肿瘤细胞。因而,从 这个意义上说,通过激活、修复、改构、甚至重建患者 抗肿瘤免疫细胞反应的治疗方法,尤其肿瘤细胞免疫 治疗,天然具有精准治疗的特征。通过激活患者体内 残存肿瘤特异性T细胞的治疗方式,已被证实具有良 好的临床疗效。更值得庆幸的是,不同T细胞所 携带的TCR受体千差万别,具有高度的多样性,为实 施针对不同肿瘤变异信息的精准医学治疗提供了足 够的广度。而且,免疫细胞来源于患者自体,作为一 种“活的药物”,具有自主性与自我适应能力,能有效 缩短开发时间。因而,精准细胞免疫治疗有望成为 肿瘤精准医疗的一个重要突破口。
本文定义的精准细胞免疫治疗是通过高通量基 因测序及大数据分析,获得针对癌细胞特异性新抗 原(neo-antigens)和具有高效应的精准T细胞(precision T cell for neoantigen,简称为PNA-T),富集 PNA-T细胞对肿瘤患者进行精准免疫治疗。涉及的 步骤(图1)包括:(1)基因检测:高通量基因检测手 段获取患者的癌细胞特有的基因变异信息(包括突 变、融合基因等),从中筛选出能高效激活免疫反应 的肿瘤特异性新抗原,这种新抗原可以来自细胞核、 细胞质、细胞膜任何部位;(2)免疫靶点的筛选:根 据患者的主要组织相容性复合体(major histocom patibility complex,MHC)分型,寻找能引发强烈免疫 的新表位(neo-epitopes);(3)寻找并富集针对新抗 原表位的PNA-T细胞:主要通过负载新表位的树突 状细胞(dendritic cells,DC)刺激,标记后的MHC-新 表位耦联体流式/磁珠分选富集PNA-T细胞,克隆 PNA-T细胞的TCR基因,通过转基因修饰手段快速 获得转基因PNA-T细胞;(4)过继细胞回输治疗:大 量扩增PNA-T细胞,实施过继回输治疗,并跟踪 PNA-T细胞的变化规律与肿瘤关系。因而,肿瘤精 准细胞免疫治疗是更为个性化的免疫细胞治疗技 术,属于第三代免疫细胞治疗技术(三代免疫细胞 治疗技术的比较见表)。
虽然肿瘤精准细胞免疫治疗是一个新概念,但 该领域内研究者已进行了一些探索研究。 2013年,rosenberg领导的团队率先采用外显子测 序技术,鉴别在患者中表达的突变蛋白,并用一种 MHC分子-抗原表位亲和力算法进行模拟预测评 估,进而合成候选的抗原表位,开展免疫反应验证。 通过此方法,研究人员能快速鉴别出了在患者肿瘤 细胞上表达,能被肿瘤浸润淋巴细胞(tumor-infiltrating lymphocytes,TIL)识 别 的 突 变 抗 原 。2014 年,Rosenberg团队将该方法成功应用到临床,他们 通过高深度外显子测序技术、免疫反应功能验证,筛 选出一位转移性胆管癌患者的高频突变基因,并鉴 定到其对应的TIL克隆,通过大量扩增该TIL克隆 并实施回输治疗,使患者的病情得到有效控制。 2014年底,另一个研究团队联合应用外显子测序技 术、转录组测序技术、高通量蛋白质谱分析技术,及 MHC分子-抗原表位亲和力模拟预测技术,寻找到 能被T细胞识别从而高效激活免疫反应的多肽疫 苗,该个性化肿瘤疫苗兼具预防性疫苗与治疗性疫 苗的效能。笔者研究团队作为全国第一家获得 细胞治疗应用批文的单位,对精准医疗在免疫细胞 治疗方向中应用的重要性具有深刻的体会,前瞻性 地开展了精准细胞免疫治疗的技术开发,搭建了高 通量测序平台,采用了患者循环肿瘤细胞的富集与 单细胞分离技术、免疫新靶点的生物信息学筛选技 术等,为实施精准细胞免疫治疗打下坚实的基础。
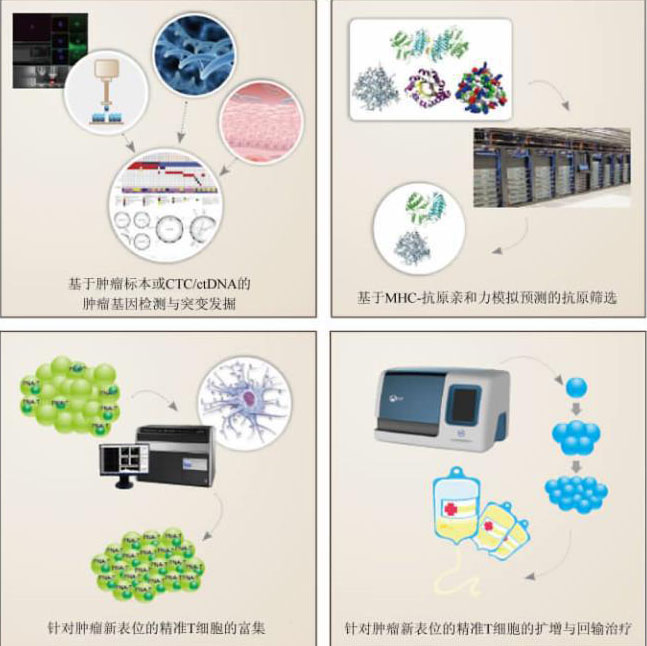
精准细胞免疫治疗的大致流程
CTC:循环肿瘤细胞(circulating tumor cell);ctDNA:循环肿瘤DNA(circulating tumor DNA);MHC:主要组织相容性复合体(major histocompatibility complex)
三代免疫细胞治疗技术的比较
如前所述,免疫治疗的理想靶点具有区别于其 他靶向治疗策略的特征:小分子靶向药物注重的是 能有效干预对肿瘤细胞生长、侵袭、转移等细胞行为 至关重要的基因及调控通路,而细胞免疫治疗的关 注点是其能否有效地被免疫系统识别,引起有效的 免疫反应。所以,同样从基因检测出发,精准细胞免 疫治疗的侧重点具有其特殊性,目前仍有几大技术 难题亟待解决。
方便快速地获取肿瘤患者的基因组变异信息
实际上,这是肿瘤基因检测所面临的共性问题。 肿瘤基因检测最直接的对象是患者原代组织标本, 但对于那些未进行过手术的肿瘤患者,肿瘤标本不 易获取,而活检穿刺的技术虽已较为成熟,但患者接 受度相对较低,尤其对那些已发生多处转移的患者。 即便之前留有标本,但往往是几个月前甚至是几年 前保存的局部标本,鉴于肿瘤基因组的动态性与异 质性,它们反映的信息已经是过时的或者代表的信 息不全。循环肿瘤细胞(circulating tumor cell,CTC)和循环肿瘤DNA(circulating tumor DNA,ctDNA)由 肿瘤发生的各个部位释放入血,能良好地反映患者 整体的肿瘤负荷、恶性程度、转移能力以及实时的基 因突变信息。因而,选择CTC和ctDNA作为基 因检测的样品来源,可以保证肿瘤治疗在取样信息 上的全面精准,且与组织活检相比具有检查微创小、 无放射性污染、经济等优点,并允许对治疗反应进行 实时监测。
然而,如何获取高纯度的CTC细胞并进行基因 测序,以及如何在外周血巨大噪音背景的情况下准 确检测ctDNA是一项具有挑战的工作。笔者研究 团队通过分离介质、抗体捕获、荧光扫描显微技术、 激光显微捕获等整合技术平台可以高效获得单个CTC细胞用于基因检测;同时开发了通过油滴PCR实现在一个油滴内单个CTC基因检测技术,以及利 用纳米孔径的芯片进行ctDNA的肿瘤突变基因检 测技术。发展类似于CAPP-Seq的超灵敏测序方 法,可以实现100%地检出2~4期NSCLC患者50%的ctDNA,可以特异性(96%)检出等位基因突变,并将错 误率降低至约0.02%水平。这类技术平台有望利 用CTC和ctDNA进行外周血肿瘤基因的精准检测, 为精准细胞免疫治疗提供可靠的检测依据。
快速准确地筛选免疫细胞治疗的适合靶点
在肿瘤表观遗传修饰变异尚无法被有效利用的现实条件下,细胞免疫治疗更多的着眼点是肿瘤基 因组遗传变异,而只有那些能形成新的氨基酸序列 且在肿瘤细胞中有效表达的变异信息才能被免疫有 效识别。因而,通过外显子组测序探知肿瘤的基因 组变异信息,通过RNA转录组测序确定发生变异的DNA的转录情况,是寻找适合免疫治疗新抗原的常 规方法。获得一系列候选新抗原信息后,如何从中 筛选出能被抗原提呈细胞有效提呈的抗原,即新表 位是至关重要的一步。通常的观点认为,能与MHC分子高效结合的抗原序列,能更好地形成MHC分 子-抗原复合物,从而具备更高的概率被提呈到细胞 表面,成为新抗原表位。因而,预测MHC分子与抗 原的亲和力是该环节的关键要素。随着MHC分子 的空间结构越来越清晰化、准确化,多种MHC分子- 抗原复合物的数学模型已被建立,通过计算机模拟 运算能预估出每种抗原与MHC分子的亲和力数 值。但鉴于MHC分子亚型的多样性,新抗原前后 氨基酸序列以不同组合、不同长度形成表位的多样 性,数据庞大,目前该预测方法仍不够成熟,假阳性 或假阴性仍普遍存在,需要后续耗时耗力的验证工 作。因而,MHC分子与抗原的亲和力的准确预估, 仍有待于通过数据的不断积累、预测模型的不断优 化来实现。
高效寻找PNA-T细胞的TCR组学技术
肿瘤精准细胞免疫治疗最终的效应细胞是PNA-T细胞。然而,虽然正常人的TCR多样性巨 大,但肿瘤患者经过长期的免疫编辑,或经过其他非 特异性治疗策略处理后,其TCR组多样性较正常人 明显降低,TCR多样性的降低预示着患者体内预留 的识别特定表位的TCR丰富度降低,即使经过上述 两个步骤成功寻找到合适的新抗原表位,但可能无 法在患者体内找到与之对应的PNA-T细胞(除非通 过转基因TCR-T技术实现),精准细胞免疫治疗仍 将以失败告终。因而,除了对肿瘤变异信息进行高 通量检测分析外,还需从免疫T细胞角度进行考 量,通过高通量测序的方法评估肿瘤患者体内是否 存留能对肿瘤抗原起反应的PNA-T细胞。目前,利 用TCR组学高通量测序技术可以较为准确地获得 患者的TCR多样性数据,可以分析肿瘤患者和正常 人之间TCR多样性的差异。但由于TCR与表位的 作用并不是一一对应关系的,即同一个TCR可以结 合不同的表位,而同一个表位也可能被不同的TCR所识别,显然它们之间亲和力会有所差异。而且TCR组库测序得到的是大量单独的α链和β链信 息,这些α链和β链理论上可以通过不同的组合构成千差万别的TCR。因而,以目前的技术水平仍难 以解析出哪个TCR-T细胞具有识别特定抗原表位 的功能。可以预想,如果通过技术进步以及抗原表 位-TCR配对大数据的积累,能最终实现获得针对特 定抗原表位,能快速地鉴别出哪些T细胞携带的TCR基因能对其有效识别并发挥治疗作用,那么将 大大缩短肿瘤免疫治疗的开发进程,为患者的治疗 赢得宝贵的时间,并可以通过测序或数字PCR等手 段检测体内这一群或者单个PNA-T细胞的克隆增 殖情况,实时监测治疗的获益情况。笔者所在团队 正致力于发展基于CTC和ctDNA为样本来源的肿 瘤抗原测序技术以及TCR组库的测序技术,包括通 过油滴技术实现高通量的单个T细胞的微乳滴PCR(emulsion-PCR,emPCR)测序来获得α链和β链的匹配信息,以此打开筛选特定抗原反应性TCR受体的方便之门。
新表位特异性PNA-T细胞的富集与扩增
实施精准细胞免疫治疗的最后阶段是PNA-T细胞克隆的富集与扩增,有三个策略可供选择:(1) 将新表位负载到患者自体的DC细胞中,然后应用 成熟的DC刺激相应的PNA-T亚群特异性增殖。该 策略的优点是相对成熟,但由于涉及DC的抗原负 载、DC内的抗原加工、DC对T细胞的提呈等一系 列过程,各个环节的技术障碍均会影响相应T细胞 的富集效率;且DC细胞扩增不易,导致该流程耗费 时间较长。(2)PNA-T细胞的直接分选。通过在体 外合成HLA-抗原肽四聚体(HLA-peptide tetramer)能够有效地被特异性T细胞识别,配合流式细胞分 选术,可以从淋巴细胞中分选出抗原特异性的T细 胞克隆,再通过成熟的T细胞培养方案可大量扩增 相应的T细胞克隆。但分选流式仪器价格昂贵,细 胞通量有限,长时间分选会影响细胞活力,对后续细 胞培养造成不良影响。为此,笔者实验室建立结合HLA-抗原肽四聚体与免疫磁珠法的T细胞分选技 术,有效提高细胞分选通量,且磁珠可通过后续切除 消除其对细胞的影响,整个流程符合临床应用规范。 下一步将设法将该技术集成到单一仪器设备中,提 高操作的便捷性与稳定性。(3)通过转基因修饰手 段,将克隆到的PNA-T细胞的TCR基因导入初始T细胞,使其快速具有识别并杀伤携带相应新表位的 肿瘤细胞的能力。
基于PNA-T细胞变化规律的疗效的实时监控 技术
疗效评估是过继细胞免疫治疗的一大技术难 点。与其他治疗方式不同,经行过继细胞治疗后,肿瘤负载可能不会立即缩小,甚至可能暂时增大,不利 于临床医生对病情的准确掌控。因而,应用PNA-T细胞对肿瘤患者实施过继细胞治疗,在治疗过程需 要跟踪血液中PNA-T细胞及其来源记忆性T细胞 的数量变化,对比观察其与肿瘤缩小或肿瘤复发、进 展的关联性,同时实时监控血液中CTC、ctDNA的含 量变化以及肿瘤新突变位点出现情况。通过数据的 积累,建立关联PNA-T细胞体内动态变化规律与患 者疗效的数学模型,从而实现医生能对病情实时作 准确判定甚至预判的目标,以作出相应治疗对策的 调整,使患者能更好地获益。
增强精准细胞免疫治疗体内疗效的辅助技术
相对于体外培养条件,肿瘤部位存在抑制免疫 的微环境。例如,肿瘤细胞表面高表达的PDL1可 与T细胞表面PD1结合,使浸润到肿瘤部位的T细 胞失能,这也是为何一些免疫检查点(如PD1/PDL1、CTLA4)单抗能通过重新激活体内残留的肿 瘤特异性T细胞,而在实体瘤的临床治疗中发挥良 好疗效的基本原理。另一方面,T胞的持续发挥 作用,需要共刺激信号(T细胞第二信号)的辅助,回 输后的T细胞如缺乏共刺激信号,将很快衰竭死 亡,这也是嵌合抗原受体(chimeric antigen receptor,CAR)T细胞技术将第一信号与第二信号偶联在同 一分子上的根本原因。因而,为有效提高精准细胞 免疫治疗的疗效,一方面可以通过免疫检查点单抗 的联合使用,阻断肿瘤微环境对回输后肿瘤特异性T细胞的不良干扰效应;另一方面,可以借助基因转 染技术,将相应的传递共刺激信号元件在体外导入 肿瘤特异性T细胞,从而延长回输后肿瘤特异性T细胞在体内的存活时间与治疗作用。在此方面,笔 者团队已建立一系列核心技术,申请中国发明专利 5项(已授权1项),显示出良好的应用价值。
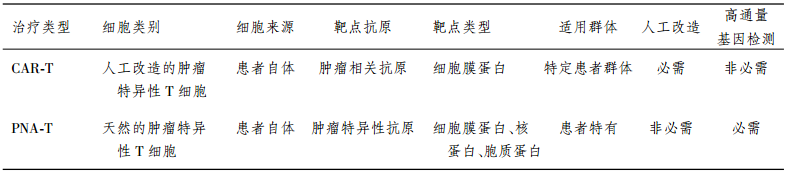
在国际上另一种热门的免疫细胞治疗技术是CAR-T细胞治疗技术。精准细胞免疫治疗与它既 具有共性特征,也有其各自的特殊属性,两者具体比 较见表。
CAR是识别肿瘤细胞膜上肿瘤相关抗原(tumor associated antigen,TAA)的单链抗体和胞内信号域 “免疫受体酪氨酸活化基序(immunoreceptor tyrosine-based action motifs,ITAM;通常为CD3ζ或FcεRIγ)”通过铰链区相连构成的嵌合基因。将CAR基因通过基因转导/转染的技术导入患者T细 胞后,使其表达CAR基因,获得的CAR-T细胞具有识别并攻击表达相应TAA的肿瘤细胞的能力。因 而,CAR-T技术本质是通过基因转染手段快速获得 肿瘤杀伤性T细胞的方法。由于CAR-T细胞所识 别的是肿瘤细胞表面的蛋白,而非与MHC分子结 合形成MHC-抗原复合物从而被提呈到细胞表面的 抗原,因而可绕过T细胞的MHC分子限制性。而 且,CAR-T技术通常将T细胞的第一信号与第二信 号偶联到同一分子结构中,使CAR-T细胞具有更强 的自主性,基本无需其他类型免疫细胞的辅助即可 发挥治疗作用。鉴于CAR-T技术的精妙设计,其具 有广阔的应用前景。
精准细胞免疫治疗与CAR-T技术所采用的效 应细胞均是患者自体的T淋巴细胞,不同之处在于 所针对的靶点类型的差别:精准细胞免疫治疗瞄准 的是患者特有的新抗原(即肿瘤特异性抗原),这些 新抗原与MHC分子结合形成MHC-抗原复合物后 被提呈到细胞表面,在被提呈前,这些抗原可以分布 在细胞各个位置,包括细胞核、细胞质、细胞膜上,可 选择范围更广。所以,不管是效应细胞本身还是所 针对的抗原,精准细胞免疫治疗均是个性化的。CAR-T技术所针对的靶点是表达在肿瘤细胞膜上 的肿瘤相关抗原,可供选择的范围较窄,特异性相对 较差;但这一抗原可以是一类患者群体中普遍存在 的抗原靶点,如表达于B细胞淋巴瘤细胞表面的CD19蛋白。因而,CAR-T技术所选用的效应细胞 来源是个性化的,但治疗靶点是非个性化的,无需通 过高通量的检测手段配合。
通常情况下,精准细胞免疫治疗策略是从患者 自体T细胞群体中寻找到天然存在的、能针对新抗 原的肿瘤特异性T细胞,归根到底采用的是未经人 工改造的天然免疫细胞;而CAR-T技术涉及转基因 过程,是人工改造的肿瘤特异性T细胞。目前,转 基因修饰技术多采用慢病毒载体系统,慢病毒为RNA病毒,其规模化生产及病毒稳定性均面临诸多 技术障碍。为解决该难题,笔者实验室已研发出一 套高效的非病毒载体系统,该非病毒载体比慢病毒 载体具有更高的转染率,具有易于规模化生产及稳 定性高的特点,将为CAR-T技术的广泛临床应用铺 平道路。
精准T细胞(PNA-T)免疫治疗与CAR-T免疫治疗的比较
随着人们对恶性肿瘤认识的不断深刻,已经越 来越意识到实施肿瘤精准医疗的必要性与迫切性。 而相对于其他基因检测指导下的个性化治疗方式, 精准细胞免疫治疗作为一种“活的药物”,为快速制 定针对特定新抗原表位的治疗方式提供足够的广度 与可行性。因而,有理由相信,精准医学的“重中之 重”是肿瘤精准医学,而中国的肿瘤精准医学突破 口在于肿瘤精准细胞免疫治疗。随着精准细胞免疫 治疗各项配套技术的日趋成熟与完善,它将在恶性 肿瘤治疗中发挥越来越重要的作用。
Human tumours typically harbour a remarkable number of somatic mutations1. If presented on major histocompatibility complex class I molecules (MHCI), peptides containing these mutations could potentially be immunogenic as they should be recognized as ‘non-self’ neo-antigens by the adaptive immune system. Recent work has confirmed that mutant peptides can serve as T-cell epitopes2, 3, 4, 5, 6, 7, 8, 9. However, few mutant epitopes have been described because their discovery required the laborious screening of patient tumour-infiltrating lymphocytes for their ability to recognize antigen libraries constructed following tumour exome sequencing. We sought to simplify the discovery of immunogenic mutant peptides by characterizing their general properties. We developed an approach that combines whole-exome and transcriptome sequencing analysis with mass spectrometry to identify neo-epitopes in two widely used murine tumour models. Of the >1,300 amino acid changes identified, ~13% were predicted to bind MHCI, a small fraction of which were confirmed by mass spectrometry. The peptides were then structurally modelled bound to MHCI. Mutations that were solvent-exposed and therefore accessible to T-cell antigen receptors were predicted to be immunogenic. Vaccination of mice confirmed the approach, with each predicted immunogenic peptide yielding therapeutically active T-cell responses. The predictions also enabled the generation of peptide–MHCI dextramers that could be used to monitor the kinetics and distribution of the anti-tumour T-cell response before and after vaccination. These findings indicate that a suitable prediction algorithm may provide an approach for the pharmacodynamic monitoring of T-cell responses as well as for the development of personalized vaccines in cancer patients.
展开The development of human cancer is a multistep process characterized by the accumulation of genetic and epigenetic alterations that drive or reflect tumour progression. These changes distinguish cancer cells from their normal counterparts, allowing tumours to be recognized as foreign by the immune system1, 2, 3, 4. However, tumours are rarely rejected spontaneously, reflecting their ability to maintain an immunosuppressive microenvironment5. Programmed death-ligand 1 (PD-L1; also called B7-H1 or CD274), which is expressed on many cancer and immune cells, plays an important part in blocking the ‘cancer immunity cycle’ by binding programmed death-1 (PD-1) and B7.1 (CD80), both of which are negative regulators of T-lymphocyte activation. Binding of PD-L1 to its receptors suppresses T-cell migration, proliferation and secretion of cytotoxic mediators, and restricts tumour cell killing6, 7, 8, 9, 10. The PD-L1–PD-1 axis protects the host from overactive T-effector cells not only in cancer but also during microbial infections11. Blocking PD-L1 should therefore enhance anticancer immunity, but little is known about predictive factors of efficacy. This study was designed to evaluate the safety, activity and biomarkers of PD-L1 inhibition using the engineered humanized antibody MPDL3280A. Here we show that across multiple cancer types, responses (as evaluated by Response Evaluation Criteria in Solid Tumours, version 1.1) were observed in patients with tumours expressing high levels of PD-L1, especially when PD-L1 was expressed by tumour-infiltrating immune cells. Furthermore, responses were associated with T-helper type 1 (TH1) gene expression, CTLA4 expression and the absence of fractalkine (CX3CL1) in baseline tumour specimens. Together, these data suggest that MPDL3280A is most effective in patients in which pre-existing immunity is suppressed by PD-L1, and is re-invigorated on antibody treatment.
展开Immune checkpoint therapy, which targets regulatory pathways in T cells to enhance antitumor immune responses, has led to important clinical advances and provided a new weapon against cancer. This therapy has elicited durable clinical responses and, in a fraction of patients, long-term remissions where patients exhibit no clinical signs of cancer for many years. The way forward for this class of novel agents lies in our ability to understand human immune responses in the tumor microenvironment. This will provide valuable information regarding the dynamic nature of the immune response and regulation of additional pathways that will need to be targeted through combination therapies to provide survival benefit for greater numbers of patients.
展开Adoptive cell therapy (ACT) is a highly personalized cancer therapy that involves administration to the cancer-bearing host of immune cells with direct anticancer activity. ACT using naturally occurring tumor-reactive lymphocytes has mediated durable, complete regressions in patients with melanoma, probably by targeting somatic mutations exclusive to each cancer. These results have expanded the reach of ACT to the treatment of common epithelial cancers. In addition, the ability to genetically engineer lymphocytes to express conventional T cell receptors or chimeric antigen receptors has further extended the successful application of ACT for cancer treatment.
展开The clinical relevance of T cells in the control of a diverse set of human cancers is now beyond doubt. However, the nature of the antigens that allow the immune system to distinguish cancer cells from noncancer cells has long remained obscure. Recent technological innovations have made it possible to dissect the immune response to patient-specific neoantigens that arise as a consequence of tumor-specific mutations, and emerging data suggest that recognition of such neoantigens is a major factor in the activity of clinical immunotherapies. These observations indicate that neoantigen load may form a biomarker in cancer immunotherapy and provide an incentive for the development of novel therapeutic approaches that selectively enhance T cell reactivity against this class of antigens.
展开Substantial regressions of metastatic lesions have been observed in up to 70% of patients with melanoma who received adoptively transferred autologous tumor-infiltrating lymphocytes (TILs) in phase 2 clinical trials1, 2. In addition, 40% of patients treated in a recent trial experienced complete regressions of all measurable lesions for at least 5 years following TIL treatment3. To evaluate the potential association between the ability of TILs to mediate durable regressions and their ability to recognize potent antigens that presumably include mutated gene products, we developed a new screening approach involving mining whole-exome sequence data to identify mutated proteins expressed in patient tumors. We then synthesized and evaluated candidate mutated T cell epitopes that were identified using a major histocompatibility complex–binding algorithm4 for recognition by TILs. Using this approach, we identified mutated antigens expressed on autologous tumor cells that were recognized by three bulk TIL lines from three individuals with melanoma that were associated with objective tumor regressions following adoptive transfer. This simplified approach for identifying mutated antigens recognized by T cells avoids the need to generate and laboriously screen cDNA libraries from tumors and may represent a generally applicable method for identifying mutated antigens expressed in a variety of tumor types.
展开Limited evidence exists that humans mount a mutation-specific T cell response to epithelial cancers. We used a whole-exomic-sequencing-based approach to demonstrate that tumor-infiltrating lymphocytes (TIL) from a patient with metastatic cholangiocarcinoma contained CD4+ T helper 1 (TH1) cells recognizing a mutation in erbb2 interacting protein (ERBB2IP) expressed by the cancer. After adoptive transfer of TIL containing about 25% mutation-specific polyfunctional TH1 cells, the patient achieved a decrease in target lesions with prolonged stabilization of disease. Upon disease progression, the patient was retreated with a >95% pure population of mutation-reactive TH1 cells and again experienced tumor regression. These results provide evidence that a CD4+ T cell response against a mutated antigen can be harnessed to mediate regression of a metastatic epithelial cancer.
展开Circulating tumor cells (CTCs) are present at low concentrations in the peripheral blood of patients with solid tumors. It has been proposed that the isolation, ex vivo culture, and characterization of CTCs may provide an opportunity to noninvasively monitor the changing patterns of drug susceptibility in individual patients as their tumors acquire new mutations. In a proof-of-concept study, we established CTC cultures from six patients with estrogen receptor–positive breast cancer. Three of five CTC lines tested were tumorigenic in mice. Genome sequencing of the CTC lines revealed preexisting mutations in the PIK3CA gene and newly acquired mutations in the estrogen receptor gene (ESR1), PIK3CA gene, and fibroblast growth factor receptor gene (FGFR2), among others. Drug sensitivity testing of CTC lines with multiple mutations revealed potential new therapeutic targets. With optimization of CTC culture conditions, this strategy may help identify the best therapies for individual cancer patients over the course of their disease.
展开The development of noninvasive methods to detect and monitor tumors continues to be a major challenge in oncology. We used digital polymerase chain reaction–based technologies to evaluate the ability of circulating tumor DNA (ctDNA) to detect tumors in 640 patients with various cancer types. We found that ctDNA was detectable in >75% of patients with advanced pancreatic, ovarian, colorectal, bladder, gastroesophageal, breast, melanoma, hepatocellular, and head and neck cancers, but in less than 50% of primary brain, renal, prostate, or thyroid cancers. In patients with localized tumors, ctDNA was detected in 73, 57, 48, and 50% of patients with colorectal cancer, gastroesophageal cancer, pancreatic cancer, and breast adenocarcinoma, respectively. ctDNA was often present in patients without detectable circulating tumor cells, suggesting that these two biomarkers are distinct entities. In a separate panel of 206 patients with metastatic colorectal cancers, we showed that the sensitivity of ctDNA for detection of clinically relevant KRAS gene mutations was 87.2% and its specificity was 99.2%. Finally, we assessed whether ctDNA could provide clues into the mechanisms underlying resistance to epidermal growth factor receptor blockade in 24 patients who objectively responded to therapy but subsequently relapsed. Twenty-three (96%) of these patients developed one or more mutations in genes involved in the mitogen-activated protein kinase pathway. Together, these data suggest that ctDNA is a broadly applicable, sensitive, and specific biomarker that can be used for a variety of clinical and research purposes in patients with multiple different types of cancer.
展开Two decades ago, the pharmaceutical industry—long dominated by small-molecule drugs—was revolutionized by the the advent of biologics. Today, biomedicine sits on the cusp of a new revolution: the use of microbial and human cells as versatile therapeutic engines. Here, we discuss the promise of this “third pillar” of therapeutics in the context of current scientific, regulatory, economic, and perceptual challenges. History suggests that the advent of cellular medicines will require the development of a foundational cellular engineering science that provides a systematic framework for safely and predictably altering and regulating cellular behaviors.
展开The immune system influences the fate of developing cancers by not only functioning as a tumour promoter that facilitates cellular transformation, promotes tumour growth and sculpts tumour cell immunogenicity1, 2, 3, 4, 5, 6, but also as an extrinsic tumour suppressor that either destroys developing tumours or restrains their expansion1, 2, 7. Yet, clinically apparent cancers still arise in immunocompetent individuals in part as a consequence of cancer-induced immunosuppression. In many individuals, immunosuppression is mediated by cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and programmed death-1 (PD-1), two immunomodulatory receptors expressed on T cells8, 9. Monoclonal-antibody-based therapies targeting CTLA-4 and/or PD-1 (checkpoint blockade) have yielded significant clinical benefits—including durable responses—to patients with different malignancies10, 11, 12, 13. However, little is known about the identity of the tumour antigens that function as the targets of T cells activated by checkpoint blockade immunotherapy and whether these antigens can be used to generate vaccines that are highly tumour-specific. Here we use genomics and bioinformatics approaches to identify tumour-specific mutant proteins as a major class of T-cell rejection antigens following anti-PD-1 and/or anti-CTLA-4 therapy of mice bearing progressively growing sarcomas, and we show that therapeutic synthetic long-peptide vaccines incorporating these mutant epitopes induce tumour rejection comparably to checkpoint blockade immunotherapy. Although mutant tumour-antigen-specific T cells are present in progressively growing tumours, they are reactivated following treatment with anti-PD-1 and/or anti-CTLA-4 and display some overlapping but mostly treatment-specific transcriptional profiles, rendering them capable of mediating tumour rejection. These results reveal that tumour-specific mutant antigens are not only important targets of checkpoint blockade therapy, but they can also be used to develop personalized cancer-specific vaccines and to probe the mechanistic underpinnings of different checkpoint blockade treatments.
展开Therapies that target the programmed death-1 (PD-1) receptor have shown unprecedented rates of durable clinical responses in patients with various cancer types1, 2, 3, 4, 5. One mechanism by which cancer tissues limit the host immune response is via upregulation of PD-1 ligand (PD-L1) and its ligation to PD-1 on antigen-specific CD8+ T cells (termed adaptive immune resistance)6, 7. Here we show that pre-existing CD8+ T cells distinctly located at the invasive tumour margin are associated with expression of the PD-1/PD-L1 immune inhibitory axis and may predict response to therapy. We analysed samples from 46 patients with metastatic melanoma obtained before and during anti-PD-1 therapy (pembrolizumab) using quantitative immunohistochemistry, quantitative multiplex immunofluorescence, and next-generation sequencing for T-cell antigen receptors (TCRs). In serially sampled tumours, patients responding to treatment showed proliferation of intratumoral CD8+ T cells that directly correlated with radiographic reduction in tumour size. Pre-treatment samples obtained from responding patients showed higher numbers of CD8-, PD-1- and PD-L1-expressing cells at the invasive tumour margin and inside tumours, with close proximity between PD-1 and PD-L1, and a more clonal TCR repertoire. Using multivariate analysis, we established a predictive model based on CD8 expression at the invasive margin and validated the model in an independent cohort of 15 patients. Our findings indicate that tumour regression after therapeutic PD-1 blockade requires pre-existing CD8+ T cells that are negatively regulated by PD-1/PD-L1-mediated adaptive immune resistance.
展开There have been no major advances for the treatment of metastatic urothelial bladder cancer (UBC) in the last 30 years. Chemotherapy is still the
标签:规模 cross 数据分析 arm zed 概率 记忆 新一代 传统
原文地址:http://www.cnblogs.com/nkwy2012/p/7967770.html