标签:
F. B. Hollinger, G. Sood
Detection of occult hepatitis B requires assays of the highest sensitivity and specificity with a lower limit of detection of less than 10 IU/mL for hepatitis B virus (HBV) DNA and <0.1 ng/mL for hepatitis B surface antigen (HBsAg). This covert condition is relatively common in patients with chronic hepatitis C virus (HCV) that seems to exert some influence on the replicative capacity and latency of HBV. Detection of virus-specific nucleic acid does not always translate into infectivity, and the occurrence of primer-generated HBV DNA that is of partial genomic length in immunocompetent individuals who have significant levels of hepatitis B surface antibody (anti-HBs) may not be biologically relevant. Acute flares of alanine aminotransferase (ALT) that occur during the early phase of therapy for HCV or ALT levels that remain elevated at the end of therapy in biochemical nonresponders should prompt an assessment for occult hepatitis B. Similarly, the plasma from patients with chronic hepatitis C that is hepatitis B core antibody (anti-HBc) positive (±anti-HBs at levels of <100 mIU/mL) should be examined for HBV DNA with the most sensitive assay available. If a liver biopsy is available, immunostaining for hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBcAg) should be contemplated and a portion of the sample tested for HBV DNA. This is another reason for optimal collection of a specimen (e.g. two passes with a 16-guage needle under ultrasound guidance). Transmission of HBV to immunosuppressed orthotopic liver transplant recipients by donors with occult hepatitis B (OHB) will continue to occupy the interests of the transplant hepatologist. As patients with OHB may have detectable HBV DNA in serum, peripheral blood mononuclear cells (PBMC) and/or liver that can be reactivated following immunosuppression or intensive cytotoxic chemotherapy, the patient needs to be either monitored or treated depending on the pretreatment serological results such as an isolated anti-HBc reaction or a detectable HBV DNA.
A 2008 international workshop on occult hepatitis B virus (HBV) infection (OBI), endorsed by the European Association for the Study of the Liver (EASL), defined OBI as the ‘presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals testing hepatitis B surface antigen (HBsAg) negative by currently available assays‘. The definition implies that infectious viral clones may be present. However, the detection of HBV DNA does not always correspond to infectivity or to the number of HBV progeny viruses released from hepatocytes. Therefore, unless infectivity has been established, clinicians should be careful in their use of terms such as OBI or occult hepatitis B viremia in deference to the more comprehensive term ‘occult hepatitis B (OHB)‘.
About 20% of OHB sera are negative for all serological markers of HBV infection except HBV DNA, 50% are positive for hepatitis B core antibody (±anti-HBs), and 35% are positive for hepatitis B surface antibody (±anti-HBc) (Fig. 1). On the basis of these HBV antibody profiles, OHB may be further stratified into seropositive or seronegative categories with the seronegative subjects being negative for both anti-HBc and anti-HBs. The HBV DNA levels are lowest in these subjects. Seropositive individuals can be further divided into two groups: anti-HBc positive, with or without anti-HBs. The HBV DNA detection rate is highest in subjects who are anti-HBc positive but anti-HBs negative, and these individuals are more likely to be infectious. When present, HBV DNA levels are intermediate in anti-HBc– and anti-HBs–positive persons. The quintessential occult HBV infection occurs in the early acute pre-seroconversion (seronegative) window period prior to the detection of HBsAg. However, OHB also may be observed in anti-HBc positive patients with chronic HBV infection following the decline of HBsAg to an undetectable level that is sometimes associated with the appearance of anti-HBs. This serological pattern occurs at a rate of 0.7–1.3% per year and is associated with older ages and hepatitis B e antibody (anti-HBe) reactivity.[3–7]
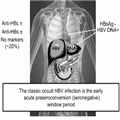
Figure 1.
Consensus definition of occult hepatitis B.
In the last decade, the application of highly sensitive molecular biology techniques has led to greater recognition and diagnosis of OHB and elucidation of its virological and clinical features. This clinical entity has been reported in healthy blood donors, patients with chronic liver disease and in patients with hepatocellular carcinoma. However, several aspects of OHB are still not resolved including the clinical significance of OBI as it relates to the risk of transmission, reactivation and progression to chronic liver disease.
The molecular basis of OHB is linked to the peculiar life cycle of HBV. A key step in replication of the virus is conversion of the 3.2 -kb circular DNA into a covalently closed circular DNA (cccDNA) in the nucleus of infected hepatocytes. The cccDNA is the template for transcription leading to production of new virions. This highly stable cccDNA is resistant to enzymatic digestion or to current antiviral agents and is the basis for persistence of HBV infection. The median cccDNA copies/hepatocyte is estimated to be approximately 1.5, but ranges from <0.01 to >50 copies/cell. These levels strongly correlate with intracellular and serum HBV DNA and are lowest in patients with occult hepatitis B.
It is estimated that an average-sized liver contains approximately 2 × 1011 hepatocytes and that 5–40% of these hepatocytes are infected at any given time.[10] Virions are assembled and released from the cell at a rate of 1–10 HBV particles/day for a total daily expression of 1010–1012 particles. Concurrently, from 103 to 105 noninfectious, subviral HBsAg particles are being released each day in excess of the virus.[11] The finding of cccDNA, RNA transcripts and pregenomic replicative RNA intermediates in the liver, peripheral blood mononuclear cells (PBMC) and/or blood of a large proportion of infected patients suggests that most occult infections are caused by low-level replication of wild-type virus.[12,13]
The reasons for persistence of low levels of HBV DNA in the absence of detectable HBsAg remain largely undefined, but it is conjectured that both host and viral factors are important in suppressing viral replication and keeping the infection under control.[14–17] Zerbini et al.[18] studied HBV-specific T-cell responses in patients with OHB, with or without anti-HBc, and identified two different profiles. Anti-HBc–positive patients showed a T-cell response typical of protective memory suggesting that this condition represents a resolved infection with immune-mediated virus control. In contrast, an HBV-specific T-cell response was not seen in anti-HBc–negative patients, suggesting the possibility that a low-level viral infection may be insufficient to allow maturation of protective memory. These results suggest different mechanisms of control of viral replication in seropositive and seronegative OHB patients.
In addition to these host responses, low levels of viral replicative activity may result from the presence of defective interfering particles or to mutations in transcription control regions or the polymerase domain leading to inefficient replication in conjunction with the discordant release of HBsAg by the hepatocytes.[17,19,20] Repression of viral transcription by cytokines induced during HBV clearance suppresses replication resulting in HBsAg negativity and low or undetectable levels of serum HBV DNA in the presence of intrahepatic HBV DNA. It also has been observed that HBsAg polypeptides are important in regulating the release of HBV and noninfectious 20-nm subviral particles from the hepatocyte. In this process, a high level of the L or large protein of HBsAg, a virion envelope component, enhances HBV morphogenesis and secretion from the hepatocyte and downregulates release of noninfectious HBsAg particles. Conversely, low levels of the L polypeptide regulate the recycling of HBV DNA-containing nucleocapsids to the nucleus and enhance the release of subviral particles from the cell. At the same time, high levels of the S or small polypeptide of HBsAg promote assembly and secretion of excess quantities of noninfectious HBsAg particles from the cell independent of virion release.
Additional mechanisms responsible for HBsAg negativity in OHB include (i) binding of HBsAg to anti-HBs to form immune complexes;[21] (ii) the development of mutations affecting the ‘a‘ epitope of the S gene that encodes amino acid residues within the major hydrophilic loop of the HBsAg coding region rendering the virus and its noninfectious particles nondetectable by current HBsAg assays; or (iii) coinfection with hepatitis delta virus or hepatitis C virus (HCV) that results in downregulation of HBV replication and a reduction in antigen synthesis.[22] This inverse correlation between the concentration of HCV RNA and HBV DNA is shown in Fig. 2.[23] Correspondingly, in transfused recipients concurrently infected with HBV and HCV, the initial appearance of HBsAg is often delayed followed by a shortened interval of HBsAg detection, a reduction in peak HBV DNA concentration and a lower peak alanine aminotransferase (ALT) level when the HBsAg first becomes positive compared to transfusion-associated HBV monoinfections[24] (Fig. 3). Previously, it was shown that HCV core protein inhibits HBV replication and gene expression,[25] but this observation has recently been challenged by Bellecave et al.[26] who observed that HBV and HCV can replicate in the same cell line without evidence for direct interference in vitro. Therefore, the HBV viral interference observed in coinfected patients is probably a result of indirect mechanisms mediated by innate and/or adaptive host immune responses.[24,27]
In earlier investigations, some individuals were diagnosed as having occult disease, when in reality a more sensitive current HBsAg assay may have revealed otherwise. On the other hand, more sensitive HBV DNA amplification assays detect occult hepatitis B at higher rates than earlier generation assays. Thus, the prevalence of OHB among various groups will change when these levels are met or exceeded. Any discussion of OHB following a review of published data therefore depends on knowing the sensitivities of the assays used in that study.
Based on current definitions of OHB, analysis of liver extracts for HBV DNA appears to be the best approach for diagnosis. But liver tissue is not always available, and standardized and valid assays for detection of HBV DNA in liver tissue are not FDA approved. Madejon and coworkers[28] found that the time between collection of the biopsy and freezing the tissue is a critical factor in preserving and detecting virus-specific nucleic acid from liver and should be <3 min.
Assays of the highest sensitivity and specificity must be used for the diagnosis of OHB. At present, the optimal standard for diagnosis is the analysis of HBV DNA extracts from plasma performed by real-time, nested polymerase chain reaction (PCR) techniques. To avoid false-negative and false-positive results, these assays should employ PCR primers that span at least three genomic regions of the HBV genome such as the S, X and core genes, and validation should require detection from at least two regions of the genome. Unfortunately, this suggestion is not usually fulfilled, and only one segment of a region is amplified. The preferred lower limit of detection (LLOD) for HBV DNA is ≤5 IU/mL or ~30 copies/mL. This is the smallest concentration of an analyte that can be distinguished from a blank or negative specimen in a single test with a 95% level of confidence. The more replicates that are performed, the more sensitive the test. This greatly enhanced sensitivity, however, amplifies the risk of contamination which then affects the specificity of these tests. Thus, the bane of these assays is that false-positive and false-negative results usually congregate around the cut-off level as a result of a Poisson distribution of the virions and the blank specimens, knowledge that healthcare workers need to consider when interpreting data in any laboratory report.
Methodology for the detection of HBV DNA has gone through several changes over the past three decades, and information concerning the relative sensitivities of these assays is important when interpreting previously published data. The first-generation tests for HBV DNA detection utilized dot-blot and liquid-phase molecular hybridization technology and measured HBV DNA in picograms per millilitre. These assays were relatively insensitive with an LLOD of approximately 283 000–425 000 geq (i.e. copies) or 1–1.5 pg of HBV DNA per mL. Adaptation of advanced molecular technologies, such as signal and target amplification, have led to the development of second-generation assays with enhanced sensitivity that have improved analytical performance characteristics, including lower limits of detection, broad linear ranges and excellent accuracy[29] (Fig. 4). A WHO International Standard for HBV DNA (97/746) was created in response to the need for standardization of these newer qualitative and quantitative assays.[30] It is a subtype adw2 genotype A isolate. In the lyophilized preparation, one international unit (IU) is equivalent to 6.31–6.42 geq using PCR-based assays. Results are now reported in IU/mL instead of genomic equivalents (geq) or copies/mL for most commercial tests, although accurate conversion factors are likely to be dependent on the chemistry used for HBV DNA quantification ranging from 5.26 to 7.3 copies/IU.[30–32] Significant variability in HBV quantification among different assays also can occur randomly despite the standardization of reporting units. Patients should therefore be monitored with the same commercial assay if the LLOD is optimal.
As a result of these advancements, target amplification assays, such as the real-time PCR assay or the transcription-based mediated amplification (TMA) assay, make possible the detection of <5 IU/mL of HBV DNA and are rapidly approaching the level of a single HBV genome. This enhancement becomes more relevant when it is recognized that the median HBV DNA level in a case of OHB is ~32–62 copies/mL or ~5–10 IU/mL (range <10–425 copies/mL) and that over 95% of the reported cases in other series have values that do not exceed 200 copies/mL[17] (Fig. 4). Thus, a majority of commercial assays currently in vogue throughout the world would miss most cases of OHB.
The current WHO International Standard for HBsAg (00/588) is a heat-inactivated product, subtypeadw2, genotype A, with a potency of 33 IU/vial.[33] In this preparation, one IU is equivalent to 5.6 Abbott ng, 1.9 French ng and 0.43 PEI units or ng confirming that ng values applied to other standards are not equivalent and some of the values have changed over time. In the United States, currently licensed HBsAg assays have highly variable cut-off values that range from <0.07 to 0.62 Abbott ng per mL of HBsAg, which is equivalent to <0.01 IU/mL. These lower limits of detection are analogous to a concentration of HBsAg found in ~2 × 107 noninfectious HBsAg subviral particles or in ~5 × 106 virions based on a virion area that is four times that of an HBsAg subunit particle.[34] Most commercial assays are designed to detect all known genotypes and subtypes of wild-type virus, but some may not recognize mutations of the S gene.[35–37] Because the ‘a‘ determinant is a target of anti-HBs selection pressure, mutations in the S gene involving amino acid substitutions within the immunodominant ‘a‘ region may affect the performance of some commercial HBsAg assays that do not focus on these anomalies. To forestall this, the more sensitive and specific commercial assays utilize monoclonal antibodies raised against the immunodominant ‘a‘ determinant of HBsAg and its variants to capture HBsAg from the blood at very low analyte levels and polyclonal antibodies to detect the HBsAg.
In regard to OHB, the cut-off for HBsAg detection based on viral load is reported to range from 720 to 11 431 copies/mL (180–2858 IU/mL assuming 4 copies or geq/mL) as shown in Fig. 4. Below these values, the HBsAg assay is usually nonreactive, whereas the reverse occurs at higher levels. This happens because of the interplay that exists between virion concentration and the number of noninfectious HBsAg particles that are released from the cell each day in excess of the virus as discussed previously. When these quantities of HBsAg (virus plus particles) are sufficient to raise the HBsAg concentration above the LLOD of the HBsAg assay, a positive result ensues. In some cases, however, there is a disconnect between the ratio of virions to subviral particles, and when this occurs discordant responses can be observed. For example, Minegishi et al.[38] using a chemiluminescent immunoassay, found that 7% of individuals whose HBV DNA levels were less than 100 copies/mL were HBsAg reactive, while 7.5% of their subjects with HBV DNA concentrations between 10 000 and 30 000 copies/mL remained HBsAg negative. Kuhns and colleagues[39] found a similar discordant response at low viral loads. From these data, it must be inferred that the ratio of HBV DNA-containing virions to subviral particles is highly variable and can range from 1:100 in patients with OHB that are HBsAg negative but have high levels of HBV DNA, to 1:100 000 or more when HBsAg is detected in association with low concentrations of HBV DNA. In each of these cases, genetic diversity of the virus also may be responsible for some of the disparity observed.
The prevalence of OHB varies significantly between geographical regions as well as among various patient populations tested.[17,40] It also depends upon the assay employed in routine serological or nucleic acid test (NAT) screening. Occult HBV infection has been reported in 0.1–2.4% of HBsAg-negative, anti-HBc–positive (±anti-HBs) blood donors in Western countries such as the United States where only 5% of the population has prior exposure to HBV and in up to 6% of a similar cohort of donors who reside in endemic areas where 70–90% of the population has been exposed to HBV.[17,41]When anti-HBc only data is evaluated (±anti-HBe), the rates range from 0% to 15% (median of 1.1%).[17,40]Correspondingly, among the general population in Asia with normal ALT levels, the prevalence of OHB has ranged from 7.5% to 16%.[42–44] In most of these studies, the prevalence of OHB is related to the presence of markers of HBV exposure or, in the case of blood donors, whether they are first-time or repeat donors. Thus, the prevalence of occult hepatitis B is higher in seropositive persons, particularly those who are positive for anti-HBc only, than in seronegative persons. In this regard, Iizuka and colleagues[45] found a positive correlation between anti-HBc titres and HBV DNA prevalence in HBsAg-negative donor blood (Fig. 5). The HBV donor yield in various parts of the world (described as the actual number of HBV DNA-positive, seronegative blood donations detected per number of donations tested employing a highly sensitive HBV NAT) currently ranges from 1 in 350 000 to 610 000 in North America, to 1 in 200 000 in Europe, to 1 in 50 000 in Japan, and to 1 in <5000 in SE Asia and Pakistan (Fig. 6). Clearly, in countries where HBV and OHB are endemic, but where anti-HBc donor screening cannot be used because of its high prevalence, NAT screening of blood donors is presumed to be necessary and very beneficial.
Among certain patient groups, the prevalence of OHB would be significantly higher if tests were performed on liver samples, but this is usually not performed. Redacted data from Brechot and colleagues[46] showed that from 25% to 67% of the patients with HBsAg-negative chronic hepatitis, cirrhosis or hepatocellular carcinoma (HCC) had detectable HBV DNA in their sera regardless of whether other HBV or HCV seromarkers were present. OHB also has been reported in 45–50% of intravenous drug users or patients with haemophilia,[47] in up to 36% of patients on dialysis,[48,49] in 8–51% of patients with human immunodeficiency virus (HIV)[50] and in approximately 30–95% of HBsAg-negative, chronic hepatitis C patients.[51,52] Castillo and colleagues[53] performed real-time PCR testing for HBV and HCV in optimally collected and processed liver biopsy samples from 76 seronegative patients with sustained abnormal liver enzymes that they had been following for up to 2 years. Within this group, 22% had OHB, 46% occult HCV and 32% had an occult coinfection (Fig. 7). Thus, 54% of these patients had intrahepatic HBV DNA suggesting that OHB should be considered in many patients with hepatitis of unknown aetiology or in those with an HCV infection.
In a recent study from Italy, OHB was found in 16.3% of 98 patients without any clinical or biochemical evidence of liver disease. These were distributed primarily among the anti-HBc–positive patients (62.5%). In another study from Japan, Marusawa et al.[54] found evidence of occult HBV infection in 13 of 14 healthy liver donors who were seropositive for both anti-HBc and anti-HBs, but in none of the three who were positive for anti-HBs alone. This included detection of episomal cccDNA and pregenomic RNA in liver tissue, which signify ongoing viral replication and risk to the recipients of these livers.
The unresolved problem in OHB is whether individuals with this disorder are at risk for progression of their disease, for transmitting infection to others, or for early death as a result of the complications of the disease. In a woodchuck model of OHB, maternal-foetal transmission to newborn woodchucks was observed from dams with occult infection[55–57] implying that vertical transmission in humans with OBI may be possible. In yearlings that have recovered from woodchuck hepatitis virus infection with the development of ‘protective‘ antibody, low levels of virus were detected in liver and PBMC, and this was accompanied by residual injury that eventually led to HCC in some.
The clinical relevance of these findings is more controversial in immunologically competent humans who have recovered from self-limited acute hepatitis B or in individuals without clinical or biochemical evidence of liver disease. As a general rule, immune individuals who have recovered from acute hepatitis B have no clinical evidence of liver disease despite the detection of traces of HBV DNA in their blood, PBMC and/or liver decades later.[1,14,16,54] In support of this, Norman and colleagues[58] determined the relative risk of HCC in 567 individuals who had developed overt or inapparent hepatitis B 40 years previously following exposure to HBV-contaminated yellow fever vaccine. As shown in Fig. 8, no significant excess mortality for HCC was observed in these two groups of patients. In contrast, Yuki et al.[59] found that liver fibrosis and mild inflammation persisted in eight patients followed a median of 7.2 years after the onset of acute hepatitis B. In another study, however, two patients who had HBV DNA in their liver following self-limited acute hepatitis B 30 years earlier were found to have only minor inflammation without fibrosis although one of these patients had a slightly increased ALT level.[60] It is comforting to note that despite these observations, no transmission of HBV has ever been demonstrated in blood donors who developed anti-HBc and anti-HBs following acute hepatitis B.[17,61] The continuous stimulation by viral replication and gene expression in this population appears to be responsible for the persistence of virus-specific cytotoxic T lymphocytes, and this response appears necessary to keep the HBV infection in check in addition to maintaining the long-term persistence of anti-HBc and possibly neutralizing anti-HBs in these patients.[16]
The risk of HBV transmission through transfusion is currently related primarily to blood donations negative for HBsAg that have been collected either during the pre-seroconversion window period (WP) or during chronic OHB.[17] In the case of WP donation, HBsAg or anti-HBc screening would not be able to identify an OBI which has prompted the implementation of HBV DNA NAT screening in many countries. These tests are poised to be utilized in the future in an HIV/HCV/HBV multiplex and minipool testing format, even though this would result in only a marginal increase in blood safety, regarding HBV transmission and outcome, in low-prevalence countries such as the United States.[62] Additionally, the availability of highly sensitive and specific HBsAg and anti-HBc assays further limit the estimated value of the HBV DNA NAT for the detection of chronic OHB in these areas.[17,63] For example, in the United States, it has been reported that the current HBV donor yield ranges from 1 in 352 451 donations to 1 in 610 488 donations.[64,65] In HBV endemic regions of the world, however, HBV DNA NAT has greater potential benefit for reducing the transmission of HBV in the absence of a universal hepatitis B vaccination programme.
Transmission of HBV from a blood donor with occult hepatitis B usually induces classical hepatitis B in recipients with a full complement of seromarkers, although the incubation period may be prolonged. However, the risk of transmission appears to be negligible when concurrent anti-HBs is present in the blood above a certain level (100–200 mIU/mL). In this regard, we observed that blood containing detectable anti-HBs carries no increased risk of transmitting hepatitis B when compared with blood that lacks this antibody.[61] In another study, no posttransfusion hepatitis B was detected in recipients transfused with RBC or fresh-frozen plasma over a 7-year period from an HBsAg-negative donor with anti-HBs (>2000 IU/L), low levels of anti-HBc and intermittent low levels of HBV DNA (<10 IU/mL).[66] In an historical prospective study conducted in the 1970s, no posttransfusion hepatitis B was observed when the anti-HBs level in the donor was >15 mIU/mL regardless of anti-HBc status.[67] Prince et al.[68]inoculated three chimpanzees with serum or PBMC from three patients with OHB who were positive for anti-HBs, anti-HBc and HBV DNA (285–2023 geq/inocula) and who had developed acute self-limited hepatitis B 23–26 years previously. None of the animals developed serological, biochemical or histological evidence of hepatitis B over a 15-month follow-up period. In a subsequent study, Gerlich[69]was unable to detect any transmission of HBV to 65 platelet recipients from a hepatitis B-vaccinated plateletpheresis donor who was anti-HBs positive (>1000 IU/L) but subsequently seroconverted to anti-HBc with the appearance of HBV DNA of a different genotype and subtype from the vaccine strain. Finally, Satake and coworkers[70] in Japan found that no HBV infections occurred in 22 recipients of HBsAg-negative, HBV DNA-positive blood that contained anti-HBs compared to 10 HBV infections that occurred among 37 recipients (27%) of OHB units that were devoid of anti-HBs (Fig. 9). Table 1 lists a number of reasons why the attack rate in the latter group may not have reached 100%. These and other data[71] confirm the high risk of HBV transmission that occurs when anti-HBs–negative blood collected from OHB donors is transfused into susceptible recipients. Conversely, a negligible risk is seen when a sufficient concentration of anti-HBs is present in HBV DNA-positive units. Correspondingly, WP donations appear more likely to transmit HBV than do donations collected from patients with chronic OHB. As a group, these studies confirm the risk of transfusing OBI to susceptible individuals when anti-HBs is absent from the blood component.
The aetiological role of OHB in patients with FHF is not well defined with much data coming from the early 1990s. In three studies from North America evaluating a total of 36 patients, from 30% to 35% with apparent non-A, non-B FHF (NANB-FHF) were found to have HBV DNA in their liver but not the serum.[72–74] In contrast, no HBV DNA was detected in liver tissue obtained from 45 cases of NANB-FHF in Britain,[75] and among French patients it was less than 5%.[76] In a recent series of NANB-FHF patients in the United States, OHB was not observed in the serum of 126 patients with non-B FHF or in liver tissue from 16 of these patients.[77] It is probable that the patient populations sampled and sensitivities and specificities of the assays employed in categorizing the patients may have been responsible for these discrepancies.
The proportion of OHB among cryptogenic liver disease patients varies based on HBV endemicity and the methods used for HBV DNA and HBsAg detection. In patients with HBsAg-negative chronic hepatitis, cirrhosis or HCC, HBV DNA has been detected in serum and/or liver in approximately 19–67% regardless of the presence of other seromarkers.[17,46,53,78,79] In the OHB study by Chemin and colleagues,[78] HBV DNA was detected in the serum as well as in the liver in 15 of 50 patients (30%) with chronic hepatitis of unknown aetiology. Among these patients, the viral load was invariably less than 104 copies/mL with a median level of 400 copies/mL. Hepatitis B core antigen (HBcAg) at low levels of expression was detected in 53% of the biopsies, mainly in the nucleus and was occasionally accompanied by HBsAg. Over 50% of the patients with OHB already had severe lesions, and in 23 cases in which follow-up biopsies were available there appeared to be progression of the liver injury in 30%. In a recent study from China, HBV DNA was detected in the sera of 28% of 159 subjects with cryptogenic liver disease, and all of these patients were positive for anti-HBc.[42] As these were not prospective studies in which disease could be chronicled from the beginning, conclusions regarding progression of liver disease in patients with OHB should be judged accordingly.
The presence of OHB in chronic HCV infection is well established. Both viruses share common routes of infection, and both are transmitted parenterally. Hence, coinfection with HCV and HBV is frequent, particularly in areas of high endemicity and among individuals at high risk for parenteral infections. OHB has been reported in up to 65% of patients with HCV and is a risk factor for cirrhosis and HCC[46,80,81](Fig. 10). Cacciola et al.[51] studied the presence of HBV DNA in serum and liver tissue of patients with chronic HCV infection and found that 33% of those with HCV had evidence of OHB compared to 14% of non-HCV-infected controls. Based on a positive HBV DNA in the explanted liver, Shetty et al.[82]reported a 50% prevalence rate of OHB in a US population with end-stage chronic hepatitis C undergoing liver transplantation (29% if based on a positive serum HBV DNA with a range of 50–200 copies/mL). This rate is higher than would be expected in an area of low endemicity, but may be explained by the fact that common risk factors predispose to the acquisition of both viruses.
In contrast to the general lack of disease progression or transmissibility in self-limited acute hepatitis B, the situation appears to be different in OHB/HCV coinfection as analysed in several cross-sectional studies. Some of these studies have suggested that HBV replication accounts for many of the ALT flares that occur in patients with HCV.[27] OHB is also known to decrease the response to interferon therapy when employed in patients with chronic hepatitis C[83] and to accelerate the progression of cirrhosis, hepatic decompensation and HCC.[27,84] With regard to this, a strong association has been noted between the presence of OHB in 204 patients with chronic hepatitis C and the development of HCC when compared to HCV monoinfected patients.[81] Although there were no differences in clinical profiles observed in this population, the degree of inflammation and regeneration was greater. In addition, nuclear HBcAg and core particles were observed in 72% of the biopsies with cytoplasmic HBsAg in 8.5%. In another study from Japan,[85] OHB appeared to be an important risk factor in the development of HCC in noncirrhotic patients following the eradication of HCV with interferon. The median period from sustained virological response to the diagnosis of HCC was 94 months in patients with OHB and 22 months in those without OHB but in whom cirrhosis was the predominant liver disorder. It has been speculated that the disappearance of HCV might upregulate HBV replication.[86] In a similar study from North America, HBV DNA, sometimes in the form of replicative intermediates (cccDNA), was detected by PCR in HCC and/or in adjacent nontumourous liver tissue in 55% of 31 patients of which 29% were coinfected with HCV.[87] Each of these studies provides a new perspective on the possible aetiology of HCC.
In a recent study from China, HBV DNA was detected in 70% of 135 HBsAg-negative patients with HCC in the absence of chronic HCV.[42] It has been shown that anti-HBc positivity is a risk factor for the development of HCC in OHB patients with alcoholic cirrhosis in the absence of chronic HCV[88] as well as in HCV-related HCC.[84,89] Most of these anti-HBc–positive individuals have a latent episomal form of HBV infection accompanied by ongoing viral replication.[89] Despite these observations, it remains controversial as to whether very small amounts of HBV DNA maintain their oncogenic potential in the absence of HCV.
OBI often leads to HBV transmission and subsequent infection during organ transplantation. In hyperendemic areas where organ shortage does not permit rejection of anti-HBc–positive organ donors, clinicians often cannot distinguish between de novo HBV infection and reactivation. In areas with a low HBV prevalence, the introduction of vaccination programmes and strict donor selection policies have the potential to reduce the risk of HBV infection. Liver grafts from donors who are HBsAg negative but anti-HBc positive can transmit HBV to susceptible recipients after transplantation at an incidence rate of 17–94%.[90–93] It is not clear, however, whether such an infection is associated with severe hepatitis. Furthermore, it has not been established whether prior hepatitis B immunization with an optimal anti-HBs response (something that does not always occur in cirrhotic patients) can effectively modulate or abort the infection. Wachs et al.[94] retrospectively reviewed a series of 25 cases of de novo hepatitis B that occurred following transplantation of solid organs obtained from anti-HBc–positive donors. They found that transmission was highest and infection more severe following liver transplants when compared to kidney and heart transplants. Contrary to that experience, Douglas and colleagues[95] observed a relatively benign course of hepatitis B in a similar group of recipients with only one of five patients developing graft dysfunction after an average follow-up of more than 7 years in the absence of therapy for hepatitis B. In another series, Dickson et al.[90] evaluated the risk of acquiring an HBV infection among liver transplant recipients from anti-HBc–positive donors and found that hepatitis B developed in 78% of 23 recipients. In these patients, the disease was often subclinical or mild and less severe when compared to hepatitis B acquired as a result of recurrent disease. Nearly 50% of infected patients had normal serum aminotransferases, and 85% had minimal inflammatory activity on liver biopsy 1 year following liver transplantation.
In a recent study from the United States, the prevalence of OBI was assessed prospectively in patients with HCV-related cirrhosis undergoing liver transplantation.[82] Of the 18 anti-HBc reactive patients (±anti-HBs) who underwent orthotopic liver transplantation, 72% had detectable hepatic HBV DNA compared to 35% of the 26 patients who were anti-HBc negative (P = 0.03). While the authors implied that OHB in the explant liver was strongly associated with HCC in the explant, a reanalysis of their data showed no association existed (P = 0.36). Interestingly, there also was no evidence of overt HBV reactivation in the patients with OHB (defined as the appearance of HBsAg) during the post-transplant period spanning a median of 13.2 months although all developed serum HBV DNA.
Liver transplant recipients with serological evidence of past infection to hepatitis B (anti-HBc positive) may have reactivation of OHB under immunosuppression in the post-transplant period. In two retrospective studies, reactivation of HBV was mild in the transplant recipients with previous serological immunity without any impact on patient or graft survival.[96,97] None of these recipients received any prophylaxis after transplantation.
As previously discussed, transmission of HBV after kidney and heart transplantation from an anti-HBc–reactive donor occurs at a much lower rate. Combining the results of four separate series, only one in 132 kidney recipients and none of 19 heart recipients from anti-HBc–positive donors became HBsAg positive.[98] In a single haemodialysis unit in Italy,[99] HBV DNA was detected in 27% of 128 HBsAg-negative haemodialysis patients that increased to 72% when anti-HBc was present in isolation. Rates were even higher when anti-HCV also was present. In a study from Turkey,[100] 15% of 138 haemodialysis patients had OHB. In their population, anti-HCV was not a contributing factor. In contrast, in a North American study, OHB was seen in only 9 of 239 patients (3.8%) on haemodialysis with a median viral load of 40 copies/mL (range 102–104 copies/mL).[48] Surprisingly, 7 of the 9 had a G145R mutation and most had been vaccinated. In another study, OHB was detected in 3.3% of 300 patients on the waiting list for kidney transplantation.[101] It was more common in Asians and African-Americans than in Caucasians and in those with chronic hepatitis C as found in other studies. The data on the prevalence and impact of OBI in renal transplant patients are limited, but the prevalence seems to be low.[102] As in most investigations, follow-up is suboptimal, but the introduction of immunosuppressive therapy seems to be less of an issue than it is in patients with HBsAg-positive hepatitis.
There is ample proof that immune suppression poses a significant risk for HBV reactivation in HBsAg-positive patients receiving chemotherapy, and there is consensus that these patients require prophylaxis with an antiviral agent.[103] In this regard, a recent meta-analysis reported a combined rate of 50% reactivation (range 24–88%) occurring in HBsAg-positive patients who did not receive prophylaxis. In contrast, the risk of HBV reactivation in OHB is less certain and depends on a number of factors. In a recent study from Japan, de novo HBV reactivation (reverse seroconversion) was observed in 4.2% of 48 HBsAg-negative, anti-HBc–positive patients who had received intensive chemotherapy including steroid-containing regimens plus rituximab for lymphoma.[104] Dhedin et al.[105] observed HBV reactivation after allogeneic–haematopoietic stem cell transplantation (HSCT) in 10.8% of 37 HBV immune patients in France with no reactivation in patients who received human stem cells (HSC) from anti-HBs–positive donors. A gradual loss of anti-HBc and anti-HBs preceded reactivation of HBV in these patients. In a large study from Hong Kong, Hui et al.[106] reported that only 8 (3.3%) of 244 HBsAg-negative malignant lymphoma patients, following completion of chemotherapy primarily with rituximab plus a steroid-containing regimen, developed HBV reactivation. All 8 of the patients with reverse seroconversion had pretreatment OBI by PCR with low viral loads that ranged from 22 to 84 copies/mL (median 68 copies/mL). A 100-fold increase in serum HBV DNA preceded the onset of hepatitis by a median of 18.5 weeks. Three of the eight patients later developed fulminant hepatic failure, but so did 6 of the 236 patients without HBV reactivation. Hui et al.[107] also investigated OHB in HSC donors in a highly endemic area and found that 19 (15.3%) of 124 donors were HBV DNA positive with a median viral load of 32–41 copies/mL (range 12–412 copies/mL) depending on whether the primers were targeted to the core region or the X region. Three of the 124 HSCT recipients developed HBV-related hepatitis 5–7 months later. Two of these had received a transplant from a donor with OBI (10.5%) vs one case following transplantation from a donor without OBI (1%; P = 0.06).
Reactivation with or without liver disease is lowest in recipients of HSC from donors who are immune[108]and highest in anti-HBc–positive and anti-HBs–negative allogeneic HSCT patients receiving immunosuppressive therapy (Fig. 11).[109] Conversely, HBV reactivation is unlikely to occur in immunosuppressed OHB patients whose anti-HBs concentration is >100 IU/L regardless of anti-HBc reactivity, and when it does occur the clinical presentation is more benign. Clinicians should recognize that while reactivation may develop during chemotherapy, it more often appears after chemotherapy has been completed. Patients with OBI with cirrhosis need stringent monitoring because the icteric hepatitis fatality rate following reactivation approaches 5–40% in these patients.
Various mechanisms of HBV reactivation are possible. First, immunosuppression during treatment with cytotoxic agents and rituximab may allow enhanced HBV replication and lead to direct hepatic toxicity. Second, the administration of a cytotoxic/immunosuppressive agent may repress the function of T cells and/or deplete B cells. Onozawa et al.[110] studied HBV seromarkers in 14 allogeneic HSCT patients with pretransplant anti-HBs and observed a progressive decrease in titre in all cases while on intensive chemotherapy with an estimated anti-HBs disappearance in 75% by 2 years and 100% at 5 years. Reverse seroconversion occurred in about two-thirds of these patients. Third, the suppressed immunological response also leads to widespread infection of HBV in hepatocytes. Following rapid withdrawal of cytotoxic agents, a rebound in the cytotoxic T-cell response is induced that can target hepatocytes presenting viral antigens leading to cellular injury.
Use of antiviral agents as prophylaxis against HBV in HBsAg-seropositive patients undergoing cytotoxic chemotherapy is becoming a standard strategy.[103,111,112] But for patients with OHB and those who are serologically HBV DNA negative but anti-HBc positive (anti-HBs <100 mIU/mL) and who are receiving corticosteroids with B and T-cell antibodies, guidelines for the use of antiviral agents have not been established. However, consensus expert opinion indicates that current data are insufficient to recommend routine prophylaxis and that antiviral therapy could be delayed until the HBV DNA becomes detectable.[111,113,114] For those with OHB, especially in the absence of anti-HBs, evidence from the studies cited above indicates that a prudent therapeutic approach would be to initiate HBV antiviral therapy (lamivudine, telbivudine, adefovir, entecavir or tenofovir) prior to commencing chemotherapy. This should be continued for at least 6 months or more after stopping the immunosuppressive treatment, especially if rituximab plus a steroid-containing regimen is being used. If a long-term treatment (>12 months) is anticipated, then adefovir, entecavir or tenofovir may be preferred, and if a more rapid response is needed, then entecavir or tenofovir could be considered. It should be noted that antiviral therapy is usually unsuccessful if started after the ALT becomes elevated. For those patients who are HBV DNA negative but have an isolated anti-HBc response (±low-level anti-HBs), the following approach could be considered based on the kinetics of reactivation:[106] 1) monitor at 4 -week intervals with a highly sensitive quantitative HBV DNA NAT (LLOD <10 IU/mL) and begin antiviral therapy when the result is >30 IU/mL, or 2) monitor at 4 -week intervals with a highly sensitive HBsAg assay (LLOD <0.1 ng/mL) and begin antiviral therapy when the test becomes positive. More studies are certainly needed to clarify the clinical usefulness, safety and cost-effectiveness of these strategies in OHB.
OBI is found more frequently in HIV-positive patients than in HIV-negative individuals, but its clinical significance is uncertain.[115–117] Even in the presence of anecdotal reports of reactivation during immunodepletion as acquired immunodeficiency syndrome ensues, the risk of seroreversion appears to be low; therefore, it does not justify any prophylaxis.
Detection of occult hepatitis B requires assays of the highest sensitivity and specificity with a lower limit of detection of less than 10 IU/mL for HBV DNA and <0.1 ng/mL for HBsAg. This covert condition is relatively common in patients with chronic HCV that seems to exert some influence on the replicative capacity and latency of HBV. Detection of virus-specific nucleic acid does not always translate into infectivity, and the occurrence of primer-generated HBV DNA that is of partial genomic length in immunocompetent individuals who have significant levels of anti-HBs may not be biologically relevant. Acute flares of ALT that occur during the early phase of therapy for HCV, or ALT levels that remain elevated at the end of therapy in biochemical nonresponders should prompt an assessment for occult hepatitis B. Similarly, the plasma from patients with chronic hepatitis C that is anti-HBc positive (±anti-HBs at levels of <100 mIU/mL) should be examined for HBV DNA with the most sensitive assay available. If a liver biopsy is available, immunostaining for HBsAg and HBcAg should be contemplated and a portion of the sample tested for HBV DNA. This is another reason for optimal collection of a specimen (e.g. two passes with a 16-guage needle under ultrasound guidance). Transmission of HBV to immunosuppressed orthotopic liver transplant recipients by donors with OHB will continue to occupy the interests of the transplant hepatologist. Correspondingly, as patients with OHB may have detectable HBV DNA in serum, PBMC and/or liver and can be reactivated following immunosuppression or intensive cytotoxic chemotherapy, the patient needs to be either monitored or treated depending on the pretreatment serological results such as an isolated anti-HBc reaction or a detectable HBV DNA.
标签:
原文地址:http://www.cnblogs.com/biopy/p/4294615.html